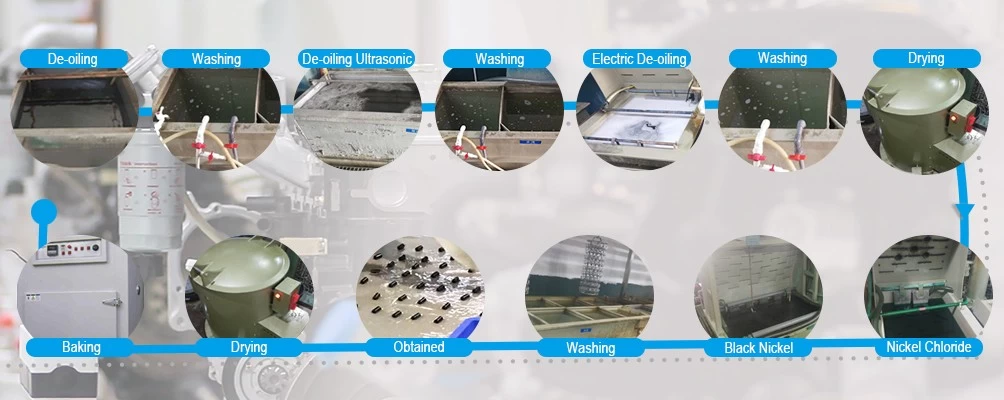
effects of electroplating
Coolidge
www.diecastingpartsupplier.com
2018-09-10 13:32:59
Electroplating changes the chemical, physical, and mechanical properties of the workpiece. An example of a chemical change is when nickel plating improves corrosion resistance. An example of a physical change is a change in the outward appearance. An example of a mechanical change is a change in tensile strength or surface hardness which is a required attribute in tooling industry.[10] Electroplating of acid gold on underlying copper- or nickel-plated circuits reduces contact resistance as well as surface hardness. Copper-plated areas of mild steel act as a mask if case hardening of such areas are not desired. Tin-plated steel is chromium-plated to prevent dulling of the surface due to oxidation of tin.
Electroplating, or electroless plating may be used as a way to render a metal part radioactive, by using an aqueous solution prepared from nickel–phosphorus concentrates which contain radioactive hypophosphite 32P ions.[11] Such a part might be used for the purposes of brachytherapy.
Electroplating, or electroless plating may be used as a way to render a metal part radioactive, by using an aqueous solution prepared from nickel–phosphorus concentrates which contain radioactive hypophosphite 32P ions.[11] Such a part might be used for the purposes of brachytherapy.